 Hydrogen fuel cells are already used as “clean” energy sources. Hydrogen can be produced using a number of different processes: Thermochemical processes that use organic materials, Electrolytic and Photolytic processes that split water (H2O) into hydrogen (H2) and oxygen (O2), and a more recently introduced method in which microorganisms such as bacteria and algae can produce hydrogen through biological processes. This blog discusses the use of genetically modified purple non-sulfur photosynthetic bacterium Rubrivivax gelatinosus CBS as a new platform for biological H2 production developed by C. Eckert, et al. at the National Renewable Energy Center and the University of Colorado.
Hydrogen fuel cells are already used as “clean” energy sources. Hydrogen can be produced using a number of different processes: Thermochemical processes that use organic materials, Electrolytic and Photolytic processes that split water (H2O) into hydrogen (H2) and oxygen (O2), and a more recently introduced method in which microorganisms such as bacteria and algae can produce hydrogen through biological processes. This blog discusses the use of genetically modified purple non-sulfur photosynthetic bacterium Rubrivivax gelatinosus CBS as a new platform for biological H2 production developed by C. Eckert, et al. at the National Renewable Energy Center and the University of Colorado.
Application Focus – Transformed Knockout Bacteria as a Platform for H2 Production
July 14, 2020 7:00:00 AM EDT
Application Focus – Electroporation for High-Efficiency Transformation of Chlamydomonas
June 17, 2020 3:00:00 PM EDT
 In this blog we will first overview the model system Chlamydomonas reinhardtii and methods for transforming this single celled, flagellated alga. We will provide protocol recommendations for high-efficiency square wave electroporation with generators such as BTX ECM 830, which can achieve a transformation efficiency of 2 to 6 x 103 transformants per µg of exogenous DNA. Next, we will cover protocol recommendations for exponential decay wave generators, such as the BTX ECM 630. Finally, we will compare the pros and cons and typical efficiencies of these methods to traditional glass bead transformation and electroporation via decaying square wave generators.
In this blog we will first overview the model system Chlamydomonas reinhardtii and methods for transforming this single celled, flagellated alga. We will provide protocol recommendations for high-efficiency square wave electroporation with generators such as BTX ECM 830, which can achieve a transformation efficiency of 2 to 6 x 103 transformants per µg of exogenous DNA. Next, we will cover protocol recommendations for exponential decay wave generators, such as the BTX ECM 630. Finally, we will compare the pros and cons and typical efficiencies of these methods to traditional glass bead transformation and electroporation via decaying square wave generators.
Application Focus – In Electroporation Mediated Creation of a Dendritic Cell-Based Cancer Vaccine
May 28, 2020 3:00:00 PM EDT
 This blog describes a new method for creating vaccines against tumors by Shadi-Yunger et al., 2019. Int. J. Cancer 144, 909-921. They report a novel MHC-II platform with a chimeric invariant chain, where the semi-peptide CLIP is replaced with a tumor specific peptide. This hybrid MHC-II chain facilitates the activation of CD4+ T-cells when expressed in dendritic cells (DCs). The researchers attempted to create an anti-melanoma vaccine by inserting different melanoma-associated antigens (MAAs) mRNA sequences into the MHC-II hybrid construct. Different MAAs were inserted into hybrid MHC-I and MHC-II constructs, and mRNAs were transfected into murine bone marrow derived dendritic cells (BMDCs) by electroporation using the BTX ECM 830 generator. Transfected BMDCs were injected into mice bearing melanoma tumors to assess the anti-tumor activity and T-cell activation stimulated by these modified DCs.
This blog describes a new method for creating vaccines against tumors by Shadi-Yunger et al., 2019. Int. J. Cancer 144, 909-921. They report a novel MHC-II platform with a chimeric invariant chain, where the semi-peptide CLIP is replaced with a tumor specific peptide. This hybrid MHC-II chain facilitates the activation of CD4+ T-cells when expressed in dendritic cells (DCs). The researchers attempted to create an anti-melanoma vaccine by inserting different melanoma-associated antigens (MAAs) mRNA sequences into the MHC-II hybrid construct. Different MAAs were inserted into hybrid MHC-I and MHC-II constructs, and mRNAs were transfected into murine bone marrow derived dendritic cells (BMDCs) by electroporation using the BTX ECM 830 generator. Transfected BMDCs were injected into mice bearing melanoma tumors to assess the anti-tumor activity and T-cell activation stimulated by these modified DCs.
Application Focus – In Vivo Electroporation to Study Axon Regeneration
May 13, 2020 3:00:00 PM EDT
 Regeneration of nerve cell axons is required for recovery of function after injury to the nervous system. In this post we will review a method to directly trace regenerating axons in vivo, recently published by Gao et al. 2020. Neural Regeneration Research 23, 1160-1165. The researchers performed in vivo electroporation of mouse adult sensory neurons in the ipsilateral dorsal root ganglion to transfect plasmid DNA encoding enhanced green fluorescent protein. Next, the sciatic nerve was squeezed with tweezers to create a model of sciatic nerve compression, and finally a direct time course of regenerating axon lengths was captured by confocal microscopy.
Regeneration of nerve cell axons is required for recovery of function after injury to the nervous system. In this post we will review a method to directly trace regenerating axons in vivo, recently published by Gao et al. 2020. Neural Regeneration Research 23, 1160-1165. The researchers performed in vivo electroporation of mouse adult sensory neurons in the ipsilateral dorsal root ganglion to transfect plasmid DNA encoding enhanced green fluorescent protein. Next, the sciatic nerve was squeezed with tweezers to create a model of sciatic nerve compression, and finally a direct time course of regenerating axon lengths was captured by confocal microscopy.
Application Focus - Modeling Dendritic Cell and T-Cell interactions in a 3-D Environment
April 21, 2020 7:00:00 AM EDT
Application Focus - Genetic Engineering of Brewer's Yeast with Electroporation
April 6, 2020 4:15:00 PM EDT

- In honor of National Beer Day, April 7th, the focus of this blog post is how to use electroporation for genetic engineering of brewing yeast. First, we will provide the ideal electroporation parameters for Saccharomyces cerevisiae using either ECM 630 or Gemini X2 electroporators. Then, we will highlight an example from the primary literature where this electroporation method was used with brewing yeast Saccharomyces cerevisiae ssp. carlsbergensis to modify the amount of ethanol in beer.
Electrofusion Basics - Determining Cell Fusion Efficiency
March 30, 2020 8:00:00 AM EDT
There are different ways of analyzing efficiency following an electrofusion experiment. These methods vary in terms of how soon after electrofusion you may analyze results, what types of equipment and reagents are required, and what type of information is determined. In this post we will provide an overview of a couple of analysis methods that provide some initial results just minutes after electrofusion.
Read MoreApplication Focus - High Efficiency Generation of CAR T-Cells via mRNA electroporation
December 5, 2019 3:00:00 PM EST
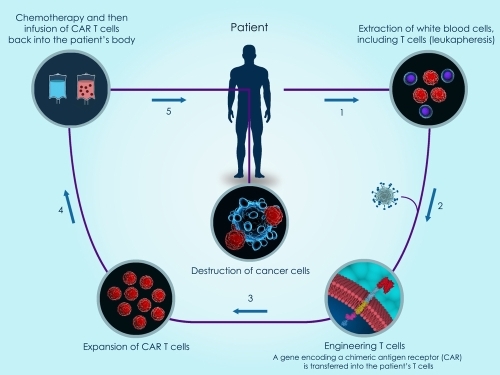
In this post we will highlight a high efficiency method for primary human T cell transfection utilizing electroporation of mRNA. This method creates genetically engineered T-cells which specifically recognize antigens of interest for CAR T-Cell immunotherapy applications.
Read MoreElectrofusion Basics - Combining Wave Forms to Create an Electrofusion Protocol
November 12, 2019 2:00:00 PM EST

The joining of membranes of neighboring cells by the application of a pulsed electrical field is called Electrofusion. Electrofusion is widely used for hybridoma creation for monoclonal antibody production, transgenics/somatic cell nuclear transfer applications, hybrid plant creation and functional studies combining two different cell types with different properties. In this post we’ll explore the types of electrical wave forms used for electrofusion and outline the way that these waveforms are combined to make a successful electrofusion protocol.
Read MoreApplication Focus – Electroporation Mediated Transfection of Human Cerebral Organoids
October 11, 2019 2:00:00 PM EDT
 In this post we will review what organoids are and how they are used to create in vitro models to study organ function and human disease states in three dimensions. We'll cover the topic of organoid transfection via electroporation in more detail. Finally, we will highlight and summarize findings reported by Ogawa et al. 2018. Cell Reports 23, 1220-1229. These researchers utilized electroporation of CRISPR/Cas9 constructs to achieve gene modification of human cerebral organoids, resulting in a glioblastoma tumor model that could be further studied in vivo in xenografted mice.
In this post we will review what organoids are and how they are used to create in vitro models to study organ function and human disease states in three dimensions. We'll cover the topic of organoid transfection via electroporation in more detail. Finally, we will highlight and summarize findings reported by Ogawa et al. 2018. Cell Reports 23, 1220-1229. These researchers utilized electroporation of CRISPR/Cas9 constructs to achieve gene modification of human cerebral organoids, resulting in a glioblastoma tumor model that could be further studied in vivo in xenografted mice.



 800-272-2775
800-272-2775
