Electrofusion Basics -
Determining Cell Fusion Efficiency
By Michelle M. Ng, Ph. D.
There are different ways of analyzing efficiency following an electrofusion experiment. These methods vary in terms of how soon after electrofusion you may analyze results, what types of equipment/reagents are required, and what type of information is determined.
In this post we will provide an overview of a couple of analysis methods that provide some initial results just minutes after electrofusion.
Typically after an electrofusion experiment, the gold standard method of assessing fusion efficiency is to dilute and plate the cells, allow for colonies to grow out, and then screen for colonies that exhibit the desired traits of the two parental cell types.
For example, in Hybridoma/monoclonal antibody production, fusion products of antibody-producing B-lymphocytes and myeloma cells undergo dilution, plating and selection by growth in HAT media for one to two weeks. HAT media supports the growth of the desired fused hybridoma cells. Next, colony counting and screening are done one to two weeks after electrofusion. The number of colonies, and more specifically the number of colonies secreting antibodies specific to the antigen of interest indicate the efficiency of the hybridoma fusion reaction.
There are, however, a couple of simple cell staining techniques that allow you view an aliquot of fused cells as soon as 30 minutes after electrofusion.
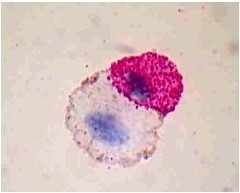
1. Nuclear Staining allows a researcher to see fused cells as evidenced by more than one nucleus per cytoplasm. For this, stain a sample of cells post-electrofusion with your desired nuclear stain. Then, visualize the cells on a microscope and check the percentage of cells that are multinucleate. The number of multinucleate cells is proportional to the efficiency of the fusion reaction; however, this method does not specifically provide information regarding how many of these multinucleate cells are the desired fusion product containing both parental cell types. Wright-Giemsa nuclear staining is a simpler method that can be done using a light microscope.
Fusion product of an immune cell and tumor cell stained with Wright-Giemsa 45 minutes after electrofusion.
You can find a protocol for Wright-Giemsa nuclear staining here.
2. Cell tracing dyes enable a researcher to specifically monitor the two parental populations of cells and their fusion products post-fusion. For this, the parental cell types may be labeled with two separate vital stains of different fluorescence spectra (such as green and red) prior to fusion. Here is an example publication where the researchers used cell tracker dyes to monitor electrofusion efficiency when fusing myeloma cells with CHO cells. This method allows you to determine how many fused cells originate from Parent A (red) + Parent B (green). The desired hybrid cells (containing both green and red) counted by this method will give you roughly one-half of the fusion efficiency numbers as the nonspecific nuclear staining method. The fluorescent staining method requires some additional cell handling pre-fusion and requires access to a fluorescence microscopy set up with at least two channels for fluorescence.
These cell staining methods are a great way to get an early measure of the success of an electrofusion experiment. They allow the researcher to compare to other experiments and proceed with confidence to clone selection and screening.
Click here to download our Hybridoma Application Note.




 800-272-2775
800-272-2775
