Frequently Asked Questions
Topics
ELECTROPORATION
-
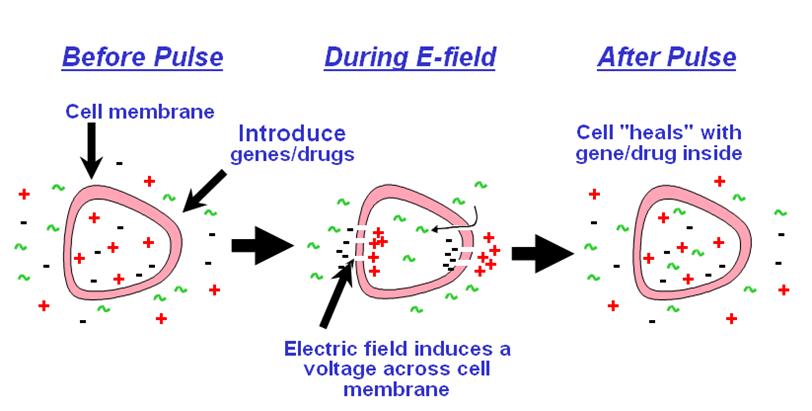
What is electroporation?
Electroporation is the application of an electrical current across a cell membrane resulting in temporary “pore” formation enabling the uptake of exogenous molecules found in the medium to either the cytoplasm (transient transfection) or into the nucleus (stable transfection), thereby transfecting or transforming the cell.
-
Why should I use electroporation?
This technique has been used to introduce plasmids such as DNA and RNA, (including siRNA) proteins, carbohydrates, dyes, and virus particles into bacterial, yeast, plant, insect and mammalian cells. Electroporation provides a highly effective alternative to other methods of transfection/transformation. Electroporation is very easy, there are no toxic side effects on the cells, the uptake of your molecule is immediate and yields greater transfection/transformation efficiencies. Electroporation, which is highly reproducible, can work with much broader cell lines or with difficult to transfect cell lines and is not limited by the size of the plasmid.
-
What is the difference between the square waveform and the exponential decay waveform?
An exponential decay wave is a waveform that is delivered then exponentially decays. This waveform is ideal when transforming bacteria and yeast. With the majority of the current being delivered immediately, the tough cell wall becomes permeable to allow the molecule of interest to enter. The square wave form differs from the exponential decay in the way it is delivered. The square wave pulse actually looks like a square. The benefit to this waveform is that it is better accepted by more delicate cells, such as mammalian cells. The square wave allows a period of homeostasis to be reached in the cells before the wave is removed. As a result, there is a lower mortality rate in cells while maintaining transfection efficiencies. While both waveforms are capable of electroporating bacterial, yeast and mammalian cells, each waveform has its benefits. Exponential decay waves will result in a higher rate of cell mortality in mammalian cells and square waves will result in lower transformation efficiencies in bacteria and yeast.
-
What system should I use for mammalian cell electroporation?
BTX offers several square wave systems that are capable of efficiently transfecting mammalian cells. The ECM 830, ECM 2001, Agile Pulse In Vivo, Agile Pulse MAX and Agile Pulse HT. The system choice will depend on what types of applications you will be running. The ECM 830 system is a general purpose mammalian cell electroporation system; it offers the researcher the ability to transfect cells in suspension in either cuvettes or 96 well plates, to perform in vivo electroporation with specialty electrodes or adherent cell transfection. The ECM 2001 was designed as a dual wave system and is primarily used for electrofusion of cells, but can also be used as an electroporator as well. The ECM 2001 offers the researcher the ability to transfect cells in suspension in cuvettes, to perform in vivo electroporation with specialty electrodes or adherent cell transfection. The Agile Pulse In Vivo system was designed for in vivo electroporation applications. It is primarily used for vaccine applications. The Agile Pulse MAX system is a large volume transfection setup for researchers working with volumes of up to 20ml. The Agile Pulse HT system is a large volume transfection setup for researchers working with volumes up to 1000ml. Call BTX sales support for help making a choice.
-
What system should I use for bacteria or yeast electroporation?
In general, we recommend using an exponential decay wave for bacteria transformation. The ECM 630 is the unit of choice to transformation bacteria and yeast. This system offers adjustable capacitance and resistance settings, the combination of which determines the time constant or time the sample is exposed to electricity. Because of the flexibility of time constant options, this system is capable of transforming both gram – and gram + bacteria, yeast, algae and plant cells in either cuvettes or in a 96 well format. BTX also offers the ECM 399 an economic exponential decay wave electroporator for simple E. coli transformation with a plasmid under 20 Kb.
-
Can we transfer settings between square and exponential decay waveforms?
Yes. Contact BTX Technical Support for a conversion chart that will guide you through the process. It is easier to convert from exponential decay waves to square waves.
-
Can I transform bacteria or yeast with a square waveform?
Yes. While the square waveform is not optimal for bacterial or yeast transformation, it can still transform the cells. That being said, the transformation efficiencies will be lower using the square waveform than with the exponential decay waveform.
-
Can I transfect mammalian cells with an exponential decay waveform?
Yes. While the exponential decay waveform is not optimal for mammalian cell transfection, it can still transfect the cells. That being said, the viability of the cells will be lower using the exponential decay waveform than with the square waveform.
-
What are the electrical variables effecting electroporation?
Waveform effects electroporation, you can choose either square waves, generally for mammalian cells or exponential decay waves, generally for bacteria and yeast. Please see section of FAQ’s discussing waveforms.
Voltage is the second key to electroporation. The voltage setting indicates how much voltage your sample will receive, this is also called Field Strength and is measured kV/cm. The higher the field strength, the better the efficiencies, however with higher field strengths come a higher cell mortality rate.
Pulse Length is the next electrical parameter effecting electroporation. Pulse length determines how long your sample is exposed to the voltage usually measured in usec, msec, and sec with a square wave generator. The combinations of Capacitance and Resistance (used in exponential decay wave generators) settings in conjunction with the resistance of your sample basically come up with the time constant.
Number of Pulses (with the square wave generators) is the number of times you want your sample exposed to the voltage. You can also choose a pulse interval for the time between pulses to allow your cells to rest. -
What other factors effect electroporation efficiencies?
There are many other factors affecting your electroporation besides electrical factors.
Cellular factors such as growth phase at time of harvesting (should be early to mid log phase) cell density, cell diameter, cell wall rigidity and susceptibility to electroporation.
There are also physiological factors such as temperature of cells, osmolarity, DNA or Plasmid concentration, quality of DNA or Plasmid, ionic concentration of buffer, and post electroporation incubation conditions.
Please contact BTX Technical Support if you need more assistance in this area. -
How do I optimize for increased electroporation efficiencies?
Optimization consists of modifying one electroporation variable at a time to reach an optimal compromise by way of transfection efficiency and viability percentage. The parameters to vary are field strength kV/cm, pulse length (msec or sec, dictated by the RC value in exponential decay wave generators), pulse number, and pulse interval. Generally, the field strength is the first variable to vary when optimizing. Please contact BTX Technical Support if you require more assistance in this area.
-
What buffers can be used during electroporation?
Researchers can use buffers with low conductivity and low osmolarity to enlarge cells and facilitate the breakdown of the membrane. The low conductivity allows users to set a lower voltage. High conductivity and high osmolarity buffers such as growth media, PBS and sucrose solution cause fairly high currents in the cuvette which could damage cells. Hypoosmolar buffers creates osmotic overpressure in the cell during electroporation. This causes water to enter the cell. The cell enlarges and the membrane expands. This leads to a reduction in the voltage applied. Furthermore, the swelling of the cell causes the cytoskeleton to break, which leads to the membrane losing its internal stability. This facilitates the electroporation of the cell and increases the efficiency of the application.
-
The results we get in our lab are not as good as they used to be. Could it be the electroporator?
Possibly. Our technical group can get you the appropriate tests to evaluate the electroporation generator you are using. If the instrument is not used for long periods of time, the capacitors in electroporation generators age and may not have the same output. In these situations, re-optimization is suggested and can usually be accomplished by varying the voltage slightly. Most reports of an efficiency decrease may be traced to changing biological parameters or technique. Electroporation is dependent on temperature, volume, buffer compositions, etc. Thus, it is wise to take a good look at these factors before exploring equipment failures.
-
My electroporator just arced. Isn’t this dangerous?
Arcing is a statistical occurrence and poses no danger to the user. The 630B safety stand will contain the arc in a closed environment. All BTX generators are "arc-proof" (short-circuit proof in the event of an arc).
-
How do I prevent arcing?
You cannot "prevent" arcing from happening, but you can minimize the probability of the occurrence by lowering the voltage or switching to a lower conductivity electroporation media. For bacterial electro-transformation, be sure to carefully follow washing procedures to remove salts. For ligation reactions dilute at least 1:5 with water or TE, or use a proven method for desalting before electroporation. Using only high-quality cuvettes can also reduce the chances of arcing.
-
How do I prepare electrocompetant E. coli?
Inoculate 1 L of rich broth appropriate for rapid cell growth with 1/10 to 1/100 volume of fresh overnight culture. Grow cells at the growth temperature and conditions optimal for the strain. Harvest when cells reach early- to mid-log phase growth (usually around OD 600 nm of ~0.5). To harvest, chill the growth flask on ice for 15-30 minutes, and centrifuge in a cold rotor at 4,000 x g for 15 minutes. Resuspend the pellets in a total of 1 L of cold water. Centrifuge as above. Resuspend in 0.5 L of cold water. Again centrifuge. If cells are to be stored frozen, resuspend in ~20 ml of cryoprotectant (usually 10% glycerol). Cells to be used fresh may be resuspended in water at this point. Centrifuge as above. Resuspend to a final volume of 2-3 ml in cryoprotectant or water. The cell concentration should be high. With some species a concentration of up to 5 x 1010 cells/ml is desirable. At this point, many species may be frozen in 10% glycerol and stored at -70° C for up to a year with little loss of electrocompetence.
• Guide to Electroporation and Electrofusion, Chang, Chassy, Saunders, and Sowers, pg. 487, Academic Press, copyright 1992. -
How many times can BTX Electroporation Cuvettes Plus be used?
BTX Electroporation Cuvettes Plus are recommended for single use only. The reason for this is that the metal inside the cuvette is oxidized during the electroporation pulse. In order to ensure consistency of results, a new cuvette is needed for each electroporation. With repeated use there is also increased risk of arcing and cross-contamination between samples.
-
What is the electrical polarity of the Tweezertrode electrodes?
The Anode (+) end of the Tweezertrode corresponds to the side of the Tweezertrode with the blue adjusting screw on it.



 800-272-2775
800-272-2775


