Application Focus - High Efficiency Generation of CAR T-Cells via mRNA Electroporation
By Michelle M. Ng, Ph. D.

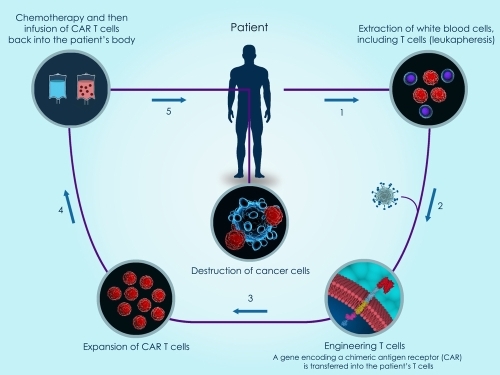 In this post we will highlight a high efficiency method for primary human T cell transfection utilizing electroporation of mRNA. This method creates genetically engineered T-cells which specifically recognize antigens of interest for CAR T-Cell immunotherapy applications.
In this post we will highlight a high efficiency method for primary human T cell transfection utilizing electroporation of mRNA. This method creates genetically engineered T-cells which specifically recognize antigens of interest for CAR T-Cell immunotherapy applications.
Immunotherapies are treatments that stimulate the body’s own immune response to fight disease. More specifically, Immuno-oncology focuses on the development of treatments that take advantage of the body’s immune system to fight cancer. Chimeric Antigen Receptor T Cells (or CAR T-Cells) are a cell-based type of immunotherapy in which engineered T-Cells are used to identify and destroy specific targets, such as tumor cells.
As a next generation method to employ, detect, and deactivate CAR T-Cells, Valton et al. have developed CubiCAR constructs. CubiCAR constructs contain an engineered CAR receptor for the T-cell to detect and destroy the target of interest, and in addition they include a CD20 mimotope that allows the engineered T-cells to be deactivated by the FDA-approved molecule rituximab. Electroporation mediated delivery of mRNA-based CubiCAR constructs into primary human T-cells resulted in 70-90% transfection efficiency and 30-100% viability for the 14 diffferent CD20 mimotopes tested in the study.
To create a cell-based immunoassay kit to monitor immune assay performance, Bidmon et al. developed a set of mRNA encoded T-Cell Receptor-engineered reference sample (TERS) control kit. The TERS kit comprises T-Cell Receptor RNA with an extended RNA stability and optimized user-specific electroporation-based manufacturing protocol to ensure consistent, high throughput creation of TERS reference samples. The group tested six electroporation devices and obtained transfection efficiencies of up to 97.3% and viabilities of up to 96% using the BTX ECM 830 and BTXpress High Performance Electroporation solution.
 In this post we will highlight a high efficiency method for primary human T cell transfection utilizing electroporation of mRNA. This method creates genetically engineered T-cells which specifically recognize antigens of interest for CAR T-Cell immunotherapy applications.
In this post we will highlight a high efficiency method for primary human T cell transfection utilizing electroporation of mRNA. This method creates genetically engineered T-cells which specifically recognize antigens of interest for CAR T-Cell immunotherapy applications.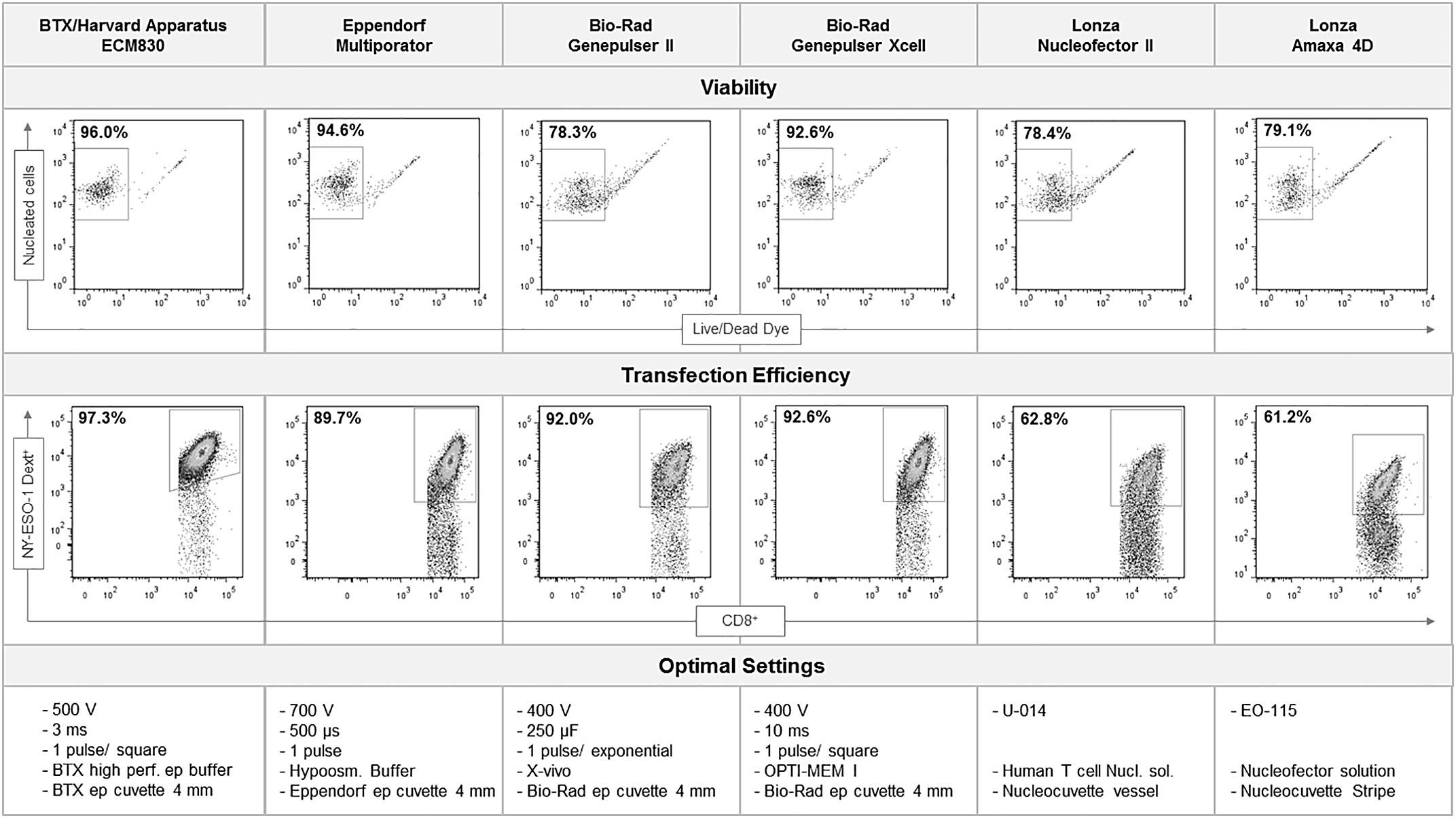



 800-272-2775
800-272-2775
