Targeting Invaders Using CAR-T Cells
By Michelle M. Ng, Ph. D.
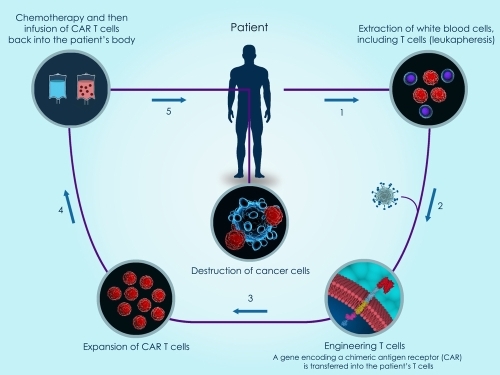
The Immune system is the body’s defense against infections and cancer. It consists of billions of cells that are divided into several different types. A subtype of white blood cells calls Lymphocytes comprise a major portion of the immune system. There are three types: B lymphocytes, T lymphocytes and Natural Killer (NK). In today’s blog post we focus on T lymphocytes (T cells) and how they work to kill tumors.
CAR-T stands for Chimeric Antigen Receptor T-Cells which are custom made T cells used to attack your target of choice. One common target is specific receptors on tumor cells. Currently a favored method is to transfect mRNA into T-cells using electroporation.
The difficulty associated with non-viral gene transfer methods in primary lymphocytes can result in low gene transfer efficiency and high transfection related toxicity. Improved transfection efficiency was achieved by electroporation using in vitro transcribed mRNA. RNA electroporation may be used to engineer T cells with new biological functions, providing a new and powerful tool for altering T cell biology where long term transgene expression is not necessary or desirable.
RNA Electroporation was found to improve transfection efficiency (90% achieved with RNA as compared with about ~50% efficiency for plasmid DNA; 1,2) and reduced transfection related toxicity. Optimization experiments using different electroporation parameters for stimulated human PBLS that viability of transfected T cells was 63-86% range 24 hours after electroporation. Cells stimulated from 2 to 18 days showed similar efficiencies indicating that the post stimulation time length does not greatly influence RNA electroporation. RNA electroporation shows advantages over plasmid DNA based electroporation. Optimized electroporation conditions did not induce adverse effects on T-cells viability and proliferation or cause apoptosis.
In conclusion, RNA electroporation is highly efficient tool to introduce genes into human and murine primary T lymphocytes. This technology can be used to engineer T cells with new biological functions.
Stay tuned as next time we tackle a 9 part series on “Designing your own optimized Electroporation Protocol”
References
- Zhao Y, Zheng Z, Cohen CJ, Gattinoni L, Palmer DC, Restifo NP, Rosenberg SA, Morgan RA. High-efficiency transfection of primary human and mouse T lymphocytes using RNA electroporation. Mol Ther. 2006 Jan;13(1):151-9. Epub 2005 Sep 2. PubMed PMID: 16140584; PubMed Central PMCID: PMC1473967. https://www.ncbi.nlm.nih.gov/pubmed/16140584
- Bell MP, Huntoon CJ, Graham D, McKean DJ. The analysis of costimulatory receptor signaling cascades in normal T lymphocytes using in vitro gene transfer and reporter gene analysis. Nat Med. 2001 Oct;7(10):1155-8. PubMed PMID: 11590441 https://www.ncbi.nlm.nih.gov/pubmed/11590441



 800-272-2775
800-272-2775
